AI systems for earlier and more accurate dementia diagnosis
Wednesday, October 29, 2025
Over the past decade, advances in medicine have saved countless lives. This is particularly true for a field like oncology, where new treatments have extended and improved the lives of millions of people who receive cancer diagnoses each year. But in other areas, the results have been less successful. As people have begun to live longer, cases of dementia have increased rapidly, with the number of diagnoses expected to triple by the year 2050. Despite efforts by researchers to develop new medicines to treat dementia, there are currently no cures.
While currently there are no medicines that can prevent or reverse dementia, it is possible to slow the progression of the disease if it is caught early, explains Salma Hassan, a Ph.D. student in Machine Learning at MBZUAI. Hassan is co-author of two new studies that hold the potential to help physicians accurately diagnose dementia and predict how the disease will evolve. Her team’s findings will be presented at the 28th International Conference on Medical Image Computing and Computer Assisted Intervention (MICCAI) held later this month in Daejeon, South Korea.
Mostafa Salem, Vijay Ram Kumar Papineni, Ayman Elsayed, and Mohammad Yaqub are co-authors of the studies.
Challenges of treating dementia
“The thing that got me interested in this field is the brain itself, the mystery of it, how little we actually know about it,” Hassan says. She is interested in developing an ‘end-to-end system’ that can help physicians address major clinical challenges related to dementia.
Dementia is an umbrella term used to describe several neurodegenerative diseases. Alzheimer’s is the most common variant, accounting for approximately 60% of all cases, with the remainder caused by vascular dementia and other subtypes.
While accurate diagnosis can help slow progression and improve a patient’s quality of life, it’s not easy to determine which subtype a patient has, and the rate of misdiagnosis can be as high as 30% in some parts of the world. The consequences of misdiagnosis are significant for patients. “If someone with vascular dementia is misdiagnosed, their life expectancy can be reduced by as much as five years,” Hassan says.
To improve the accuracy of diagnoses, Hassan and colleagues developed ClinGRAD, a multimodal heterogenous graph neural network that analyzes genomic data, magnetic resonance imaging (MRI) of the brain, and clinical information about patients, making a prediction about the type of dementia they have. The system classifies patients into four groups: healthy control, mild cognitive impairment, vascular dementia, and Alzheimer’s disease.
The code for ClinGRAD is available here.
The importance of interpretability
In healthcare, clinicians need to understand how AI systems come to their conclusions. Most AI models, however, operate like “black boxes.” Even if they are highly accurate, their results aren’t interpretable by users.
Hassan and her colleagues therefore wanted to develop an interpretable system that would foster trust with clinicians. They designed a graph neural network model that processes multimodal data while providing interpretability and preserving the biological structure of the data. To do this, the researchers integrated techniques inspired by expert clinicians who treat dementia into ClinGRAD.
When analyzing MRI data, clinicians often assess bilateral differences across brain hemispheres. They also use advanced imagining techniques, including a method called diffusion-weighted imaging (DWI) that provides insights related to the progression of dementia. And when analyzing genetic data, clinicians look at what are known as gene co-expression networks, which illustrate how genes are activated together across different regions of the brain. ClinGRAD benefits from these methods.
“Graph neural networks work best for this problem because we were able to embed the relationships between different kinds of data in a way that we couldn’t with a different architecture,” Hassan says. “The model can understand that there is already an established connection between them rather than building these relationships from scratch.”
ClinGRAD generates embeddings of the multimodal data, and the embeddings are passed through a classification layer. The class with the highest probability is the prediction produced by the model. “It identifies which nodes in the graph are the most influential and presents them to a radiologist to verify that information,” Hassan says. “It’s not about replacing clinicians with AI. It’s about increasing their speed and accuracy to aid them in their clinical work.”
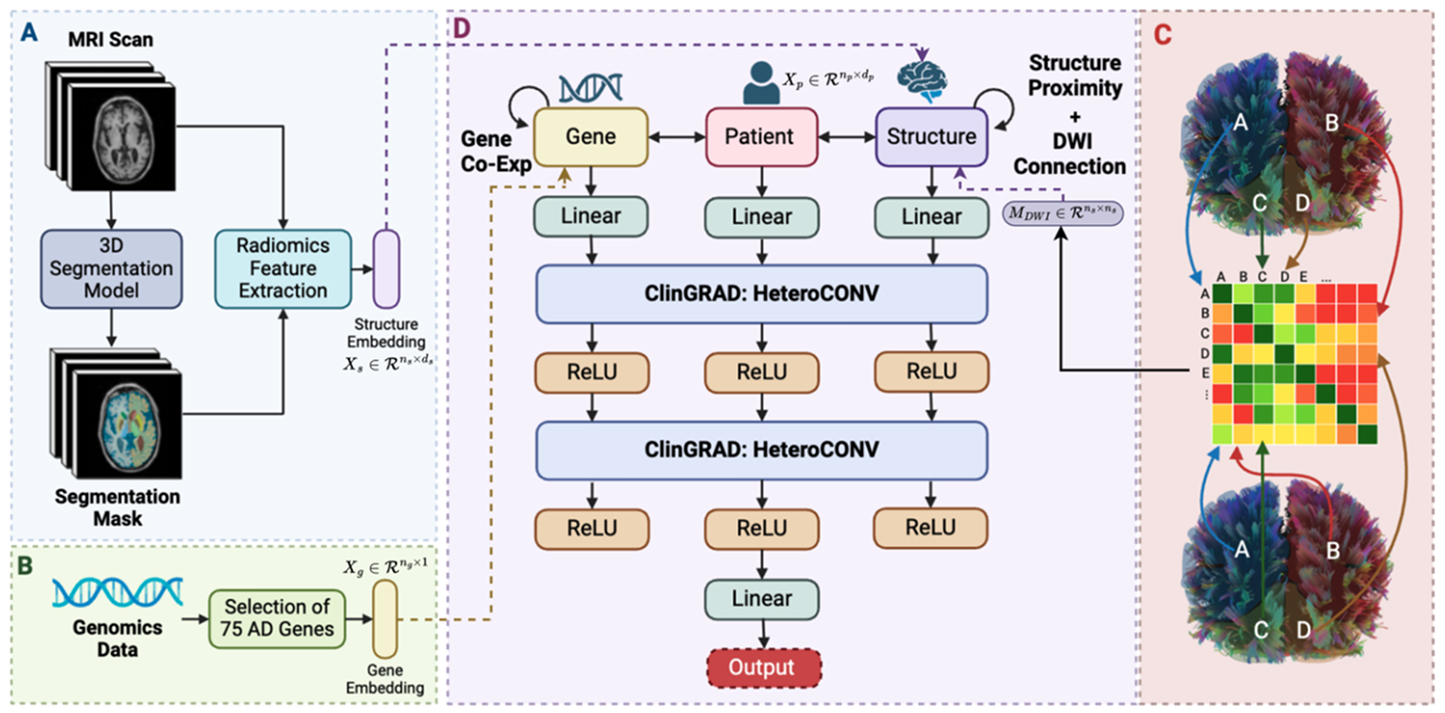
Architecture of ClinGRAD.
The researchers tested ClinGRAD on a dataset called ANMerge. ClinGRAD was highly accurate, correctly identifying 98.75% of cases. It outperformed another multimodal method (97.92%) and two single-modality approaches, one that used a 3D convolutional neural network (84.27%) and another that used a 3D vision transformer (87.12%).
In the future the researchers hope to validate ClinGRAD on datasets that are more diverse and integrate the ability to process additional kinds of biological data.
Early prediction of Alzheimer’s disease
Hassan and her colleagues developed another system called MAGNET-AD that is designed to be used long before a clinical diagnosis of Alzheimer’s is made. MAGNET-AD predicts what is known as the Alzheimer’s cognitive composite (PACC) score, a measure of cognitive function and the timepoint at which a patient will develop Alzheimer’s disease.
The system has the potential to help physicians intervene early in the development of the disease, allocating medical resources to the patients who need them most. Its code is available here.
Unlike ClinGRAD, MAGNET-AD processes data collected at different timepoints and infers the time it will take the patient to transition to full onset of Alzheimer’s disease. Hassan says that it could be used as a screening tool up to 20 years before a patient develops the condition.
Today, clinicians often use cognitive tests to determine the status of a patient. While these tests provide valuable information, it’s not always the case that a patient who has a worse score will develop Alzheimer’s faster than someone who has a better score, Hassan explains. “There are many other aspects that play a role, so you need to have a model that can understand the patterns of all of these data types as well as across time.”
MAGNET-AD is the first spatiotemporal graph neural network that predicts PACC score and time to Alzheimer’s conversion in the same model using multimodal data. Other systems have been developed, but they struggle to make sense of both longitudinal and time-invariant data.
MAGNET-AD identifies key timepoints related to disease progression while producing results that are interpretable by clinicians.
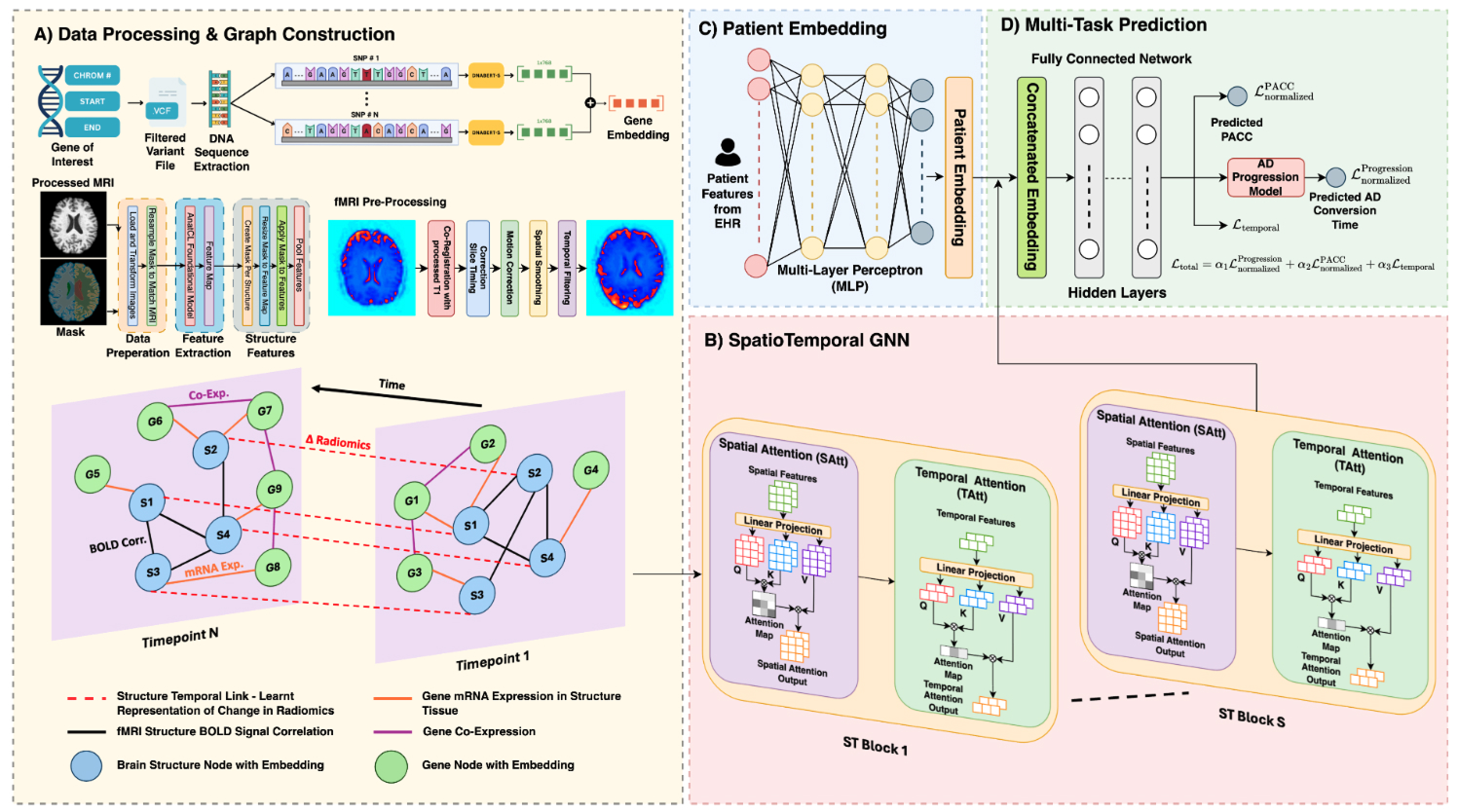
Overview of the MAGNET-AD framework that is designed for Alzheimer’s disease progression prediction and patient classification. It is the first system of its kind to use a spatiotemporal graph neural network for time-to-event prediction.
Like ClinGRAD, MAGNET-AD fuses several different data modalities together, including MRI images, functional MRI (fMRI), genetic data, and electronic health records.
The dataset that was used to train the model includes information gathered about patients, such as brain scans, collected at different time points. This timeseries data allows the system to identify patterns related to disease progression. “We can extract metrics regarding the influence of the brain structures and instead of looking at a static image we can see how it changes,” Hassan says.
The researchers compared MAGNET-AD to other deep learning approaches and commonly used prognosis models. MAGNET-AD performed better than other methods according to a metric called concordance index (C-index), which measures prognostic performance. MAGNET-AD’s C-index was 0.8582, while the next-best performing model’s C-index was 0.8041.
The researchers also tested MAGNET-AD in different configurations, including using only MRI data, using MRI and genomic data, and with and without temporal weights. It performed best when accounting for MRI and genomic data and temporal, learned radiomics, gene structure and gene-gene edge weights.
The researchers note that the impact of including temporal edge weights was “significant even in the presence of genetic information, indicating that these components capture complementary rather than redundant information about disease progression.”
Improving the lives of patients
As interesting as solving technical problems is, Hassan says that when building AI systems for healthcare, it’s always important to remember that she isn’t simply developing new ways to make sense of complex information. “These data represent real life patients who experienced this disease and we’re trying to build something better for the future,” she says.
Moreover, it takes collaboration between AI researchers and clinicians to develop systems that can have a real impact on people’s lives. “Bridging the gap between the technical side and the clinical side is only possible through collaboration.”
- healthcare ,
- medicine ,
- health ,
- MICCAI ,
- Ph.D. ,
- neural networks ,
- medical imaging ,
Related
AI foundation model GluFormer outperforms clinical standards in forecasting diabetes and cardiovascular risk
Nature-published paper with MBZUAI researchers demonstrates how AI can transform glucose data into powerful predictors of long-term.....
- digital public health ,
- cardiovascular ,
- diabetes ,
- HPP ,
- nature ,
- foundation models ,
- health ,
AI and the silver screen: how cinema has imagined intelligent machines
Movies have given audiences countless visions of how artificial intelligence might affect our lives. Here are some.....
- science fiction ,
- cinema ,
- art ,
- fiction ,
- artificial intelligence ,
- AI ,
Mind meld: agentic communication through thoughts instead of words
A NeurIPS 2025 study by MBZUAI shows that tapping into agents’ internal structures dramatically improves multi-agent decision-making.
- neurips ,
- agents ,
- machine learning ,